Built on real regulatory workflows
Not generic templates. The platform mirrors how teams actually prepare MDR/IVDR documentation —step-by-step, with structured tasks, annex-aligned folders, and clear "what to include" guidance.
Navigate MDR certification with confidence using clear pathways, expert connections, and AI support.
Best for teams in early ideation or concept stage. Clarify whether your health tech falls under MDR/IVDR.
Already have a product that needs certification? Follow our structured compliance roadmap and discover what risk classification you are in.
A Product owned by Health Tech Hub Copenhagen and backed by NNF
A simple 3-step flow from “not so sure” to a clear compliance plan.
Add key details and the intended purpose to your device description.
Follow a guided wizard that walks you through the critical decisions (MD/IVD, classification, conformity assessment route) and shows exactly what they mean for your obligations.
Instead of starting from a blank document, you get a structured plan: folders, checklists, sub-tasks, and What to include guidance - so you move faster and miss nothing important.
Trust in regulated markets is built through authority, process, and transparency.
Not generic templates. The platform mirrors how teams actually prepare MDR/IVDR documentation —step-by-step, with structured tasks, annex-aligned folders, and clear "what to include" guidance.
From day one, you work in a reviewable structure. Track progress, maintain consistency across documents, and reduce gaps that slow down internal reviews and Notified Body discussions.
Know what's required - and why. Every step helps you understand the rationale behind decisions (classification, route, key obligations), so you can align stakeholders and defend choices with confidence.
Plan, draft, and maintain your MDR documentation in one guided workflow.
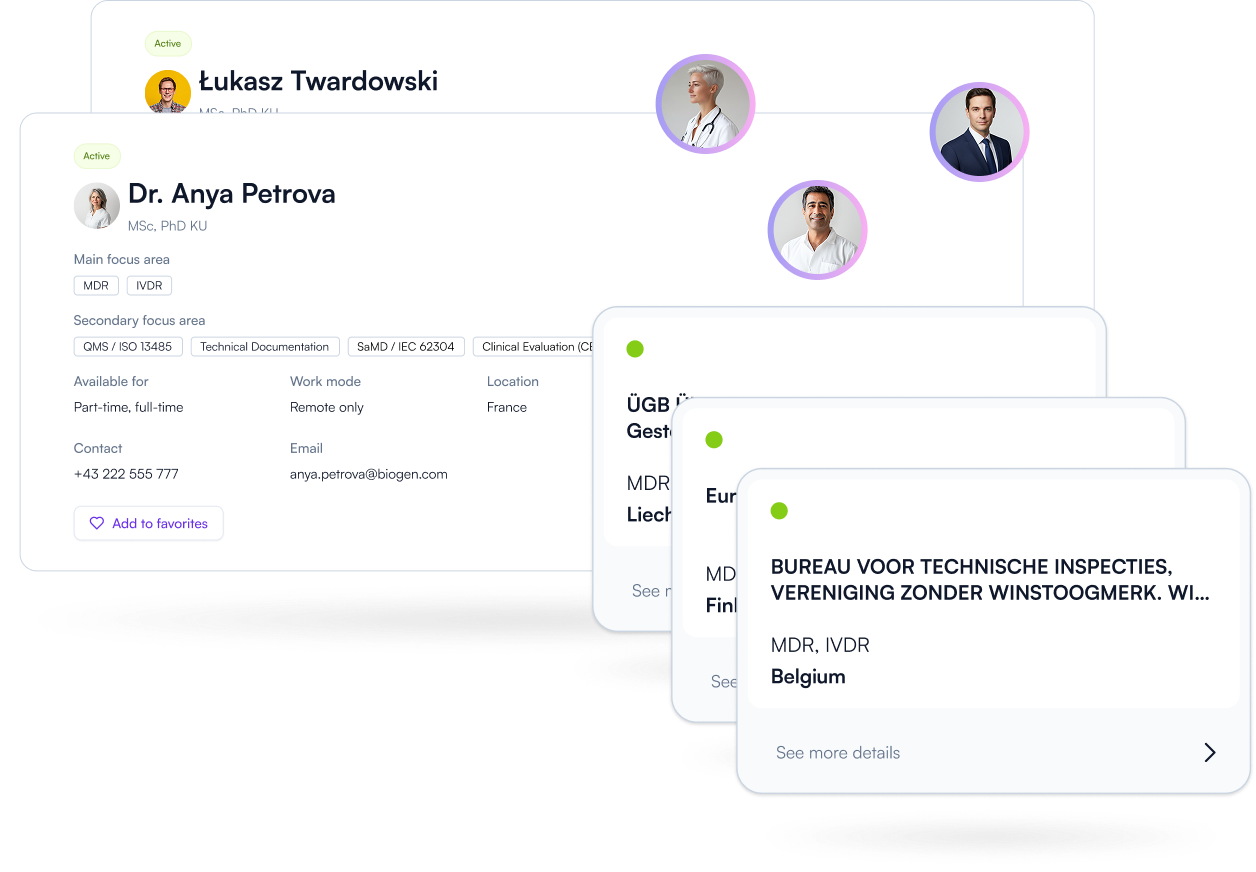
Find the right experts faster. Compare specialization, scope, and collaboration models — and connect when your project needs regulatory support. Less searching, better fit, faster progress.
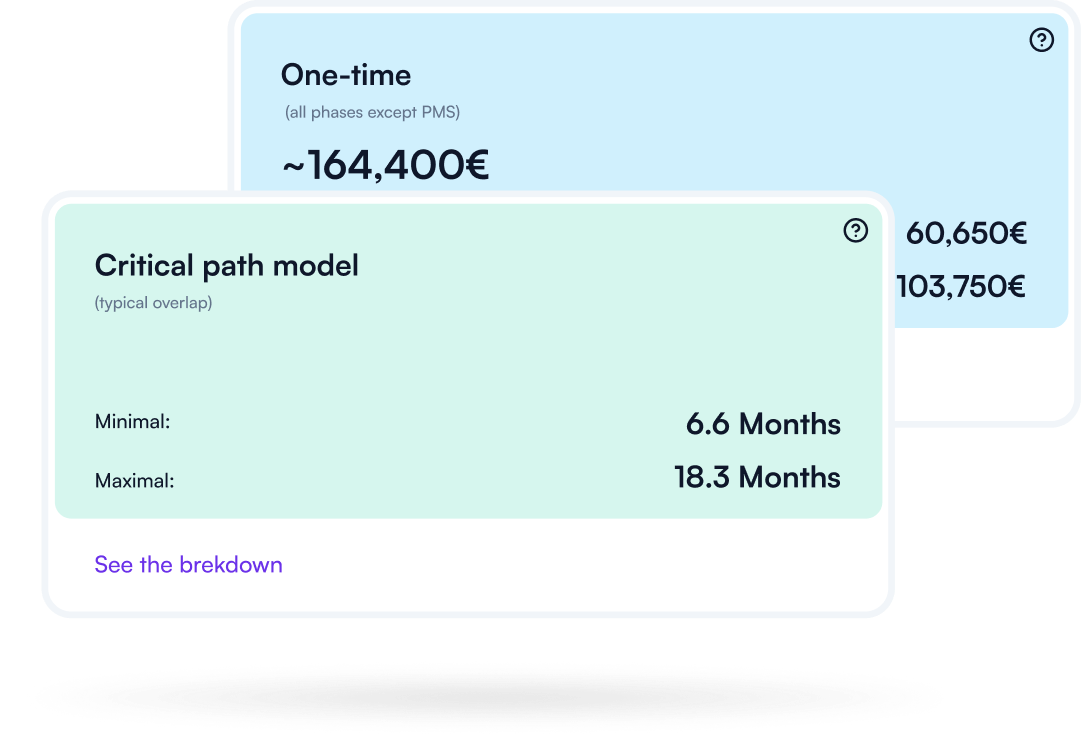
Understand what drives effort and cost across your compliance journey — so you can plan realistically, prioritize, and avoid late surprises. Make timelines and budgets defensible.
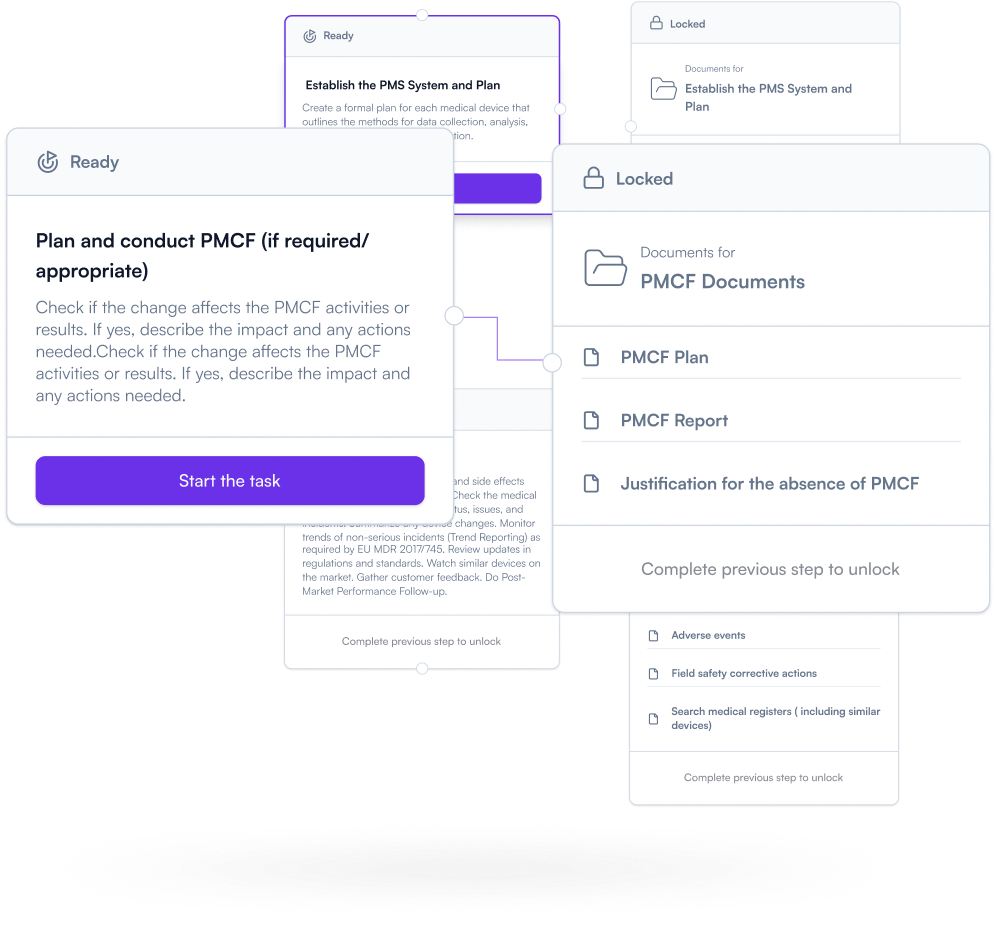
Don’t treat PMS (Post-market surveillance) as an afterthought. Plan your post-market activities early and keep documentation aligned as real-world feedback and changes come in. Keep your PMS evidence audit-ready — continuously.
Clear answers for teams building regulated products.
Create an account to continue the MDR assessment. You'll be able to view the outcome in the platform once ready.